Monitoring of Electroless Plating Baths by Capillary Electrophoresis
Ed. Note. In the past two weeks, you read about Capillary Zone Electrophoresis For Electroless Plating Bath Samples. This week, we're running a piece on the use of capillary electrophoresis for monitoring of electroless plating baths.
Electroless plating, mainly used for the plating of non-metals (i.e. ceramics and plastics), allows the plating of complex shaped parts with a uniform film thickness. In addition to metal cations, the bath solutions contain additives such as reducing agents (which drive the plating reaction) and organic acids (as buffering and/or metal complexing agents). Inorganic anions are also present as counter-ions of the plating metals. These ions can easily be monitored using capillary electrophoresis (CE) with indirect UV detection.
The Experiment
Anion analysis was performed using the HP3D Capillary Electrophoresis system (by Hewlett-Packard) equipped with diode-array detection and computer control via HP ChemStation. The analysis uses the HP Plating Bath Analysis Kit.
Prior to first use, a new capillary was flushed with run buffer for 15 minutes (at 1 bar). Between analyses, the capillary was flushed for four minutes from an extra buffer vial into waste. Buffer vials were replaced after 10 runs when using 2ml vials and after five runs when using 1ml vials. Sample preparation consisted simply of dilution with water.
The Results
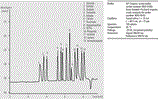
Figure 1 shows the analysis of two different plating baths. Electroless nickel-plating baths contain nickel sulfate or nickel chloride, together with hypophosphite as the reducing agent. Formate, present in the electroless copper-plating bath, is an oxidation product of Formaldehyde, which is used as a reducing agent. The assay was linear over the range 10-100 ppm with r2>0.999. The method detection limit was 1-2 ppm. For the analysis of the electroless nickel-plating bath repeatability (n = 8) was <0.1% RSD for migration times and <4.5% RSD for peak area. The assay also allows the analysis of iron (II) and iron (III) in electroplating with direct UV detection at 230 nm (data not shown).
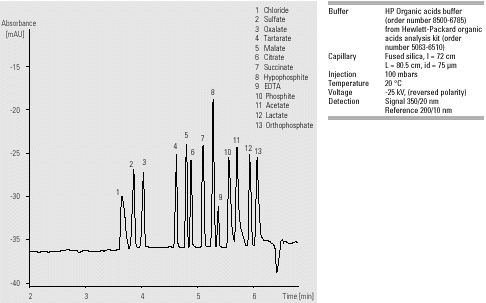
In the plating bath industry, the monitoring of additives in bath solutions or waste is essential for quality control, cost saving and environmental concerns. Electroless plating bath samples have presented a number of challenges to ion chromatography. CE, in contrast, allows a quick determination of all major components with only minor sample preparation.
By Tomoyoshi Soga, an application chemist at Yokogawa Analytical Systems, Inc., Tokyo, Japan, and Maria Serwe, an application chemist at Hewlett-Packard GmbH, Waldbronn, Germany.
For more information, please contact Lori Keelty at Agile Technologies at +49 7243 602 361, lori_keelty@agilent.com. Also, check out the Agile Technologies website: www.chem.agilent.com/cag/main.html.
