Tablet Coater Containment Systems
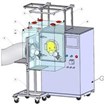
Used for lab scale and production operations at multiple International Pharma manufacturers, our contained Tablet Coater applications take the idea of retrofits to another level. Here, existing and new equipment are supported.
Used for lab scale and production operations at multiple International Pharma manufacturers, our contained Tablet Coater applications take the idea of retrofits to another level. Here, existing and new equipment are supported.
Two methods have been applied. One uses flanges that are added to the piece of process equipment by the original equipment manufacturer (OEM). The second is to totally encapsulate the coater with a pan mounted flexible enclosure.
The use of flexible containment allows the end user to process contained when needed and to existing, open processing procedures when containment is not required. In both cases, cost savings are realized by modifying an existing design or using an existing piece of equipment with no modifications.
Equipment Mounted Enclosures separate the Process and Technical Areas
The use of stainless steel flanges added to the coater enables the containment of the process area only. The enclosure is attached to the flange and includes glove sleeves, bungee cords, and HEPA filters. These features support access to the equipment while maximizing ergonomics and support operators from the 5th percentile female to the 95th percentile male.
Containment for the O'Hara LCM Tablet Coater, for example, is provided by two individual rectangular enclosures. One enclosure surrounds the pan access door and the second the exhaust plenum access door. Details of this application are seen in the Flexible Containment Solutions Guide (FCSG) 010.
Pan Mounted Enclosures Encapsulate the Entire Coater
Pan mounted enclosures allow the entire piece of process equipment to be contained. This is beneficial for equipment that can not be modified to use the flange mounted technology described above.
Again, the enclosure is supported by bungee cords, attached to the pan, and includes glove sleeves for access to the equipment. The enclosure "moves" with the operator, as is the case with all of our flexible enclosure systems, to maximize ergonomics as noted above.
Transfer Sleeves Support Contained Transfer of Fragile Tablets
Transfer Sleeves are employed on the Thomas Accela-Cota® application shown. In this case, contained transfer is achieved by attaching ILC's patented multiple o-ring canister on the bottom of the IBC and the inlet of this purpose built coater.
A dual neck transfer sleeve attaches to the canisters. The outer neck provides containment while the inner neck functions as a decelerator to "ease" the tablets into the coating pan.
Materials
The enclosures are manufactured from clear ArmorFlex® 113 film that will allow room light to illuminate inside the enclosure for easy viewing. This rugged film provides a safe working environment while enabling the enhancements developed through numerous installations using this flexible containment technology.
The Transfer Sleeves are also made from another version of ArmorFlex?® film. This is the same monolayer film used in our DoverPacs®.
What are the Features and Benefits of this technology?
Features
- Retrofit to existing equipment design
- Process and Technical areas separated
- Validated containment technology
- Clear film
- Passive system
- Flexible materials
- Disposable components
- Adaptable to other particle size reduction equipment
Benefits
- Provides the lowest overall cost of process ownership through low capital and operating cost including reduced cleaning and cleaning validation
- Fastest turnaround of a processing suite for subsequent manufacturing campaigns
- Process is contained without contamination of motor, drive shaft, and controls
- Nanogram containment levels achieved
- Supports visibility for maintenance
- Does not affect ATEX and Ex ratings
- Ergonomics maximized
- Speed of implementation
What containment level provided?
OEB 5 with results in the nanogram range. This is based on customer test data, other proven applications, third party testing to the "SMEPAC" protocols on similar designs, and the 100% inflation tests performed on the deliverable systems.
Occupational Exposure Levels above are in µg/m3.
Why use this over other technologies?
The cost of ownership, ergonomic advantages, and speed of delivery benefits of this flexible solution far outweigh those of rigid isolation systems.
Tools such as Lean Manufacturing come into play more and more. For example, the time to clean and validate the cleaning are major bottlenecks for processing efficiencies in the plant. Being able to minimize this part of the process results in getting products to market faster and at an overall reduction in operating costs when considering labor, utilities, and waste disposal costs. It also supports getting multiple products to market faster within an existing facility without risking product safety.
Click Here To Download:
•Flexible Containment Solutions Guide: Mill Containment System - Bohle BTS 100
•Flexible Containment Solutions Guide: DoverPac® SF
•Flexible Containment Solutions Guide: Drum Transfer System
•Flexible Containment Solutions Guide: Mill Containment System - Underdrive Design
•Flexible Containment Solutions Guide: Bag In/Bag Out System
•Flexible Containment Solutions Guide: Drum Sampling Enclosure System
•Flexible Containment Solutions Guide: Tray Dryer Enclosure Technology
•Flexible Containment Solutions Guide: Tablet Coater Containment System – O’Hara LCM Tablet Coater
•Flexible Containment Solutions Guide: Contained Offloading Of Aurora
•Flexible Containment Solutions Guide: Coaxial Neck DoverPac®
•Flexible Containment Solutions Guide: Document Transfer Enclosure
•Flexible Containment Solutions Guide: Mill Containment System - Jet Mills
